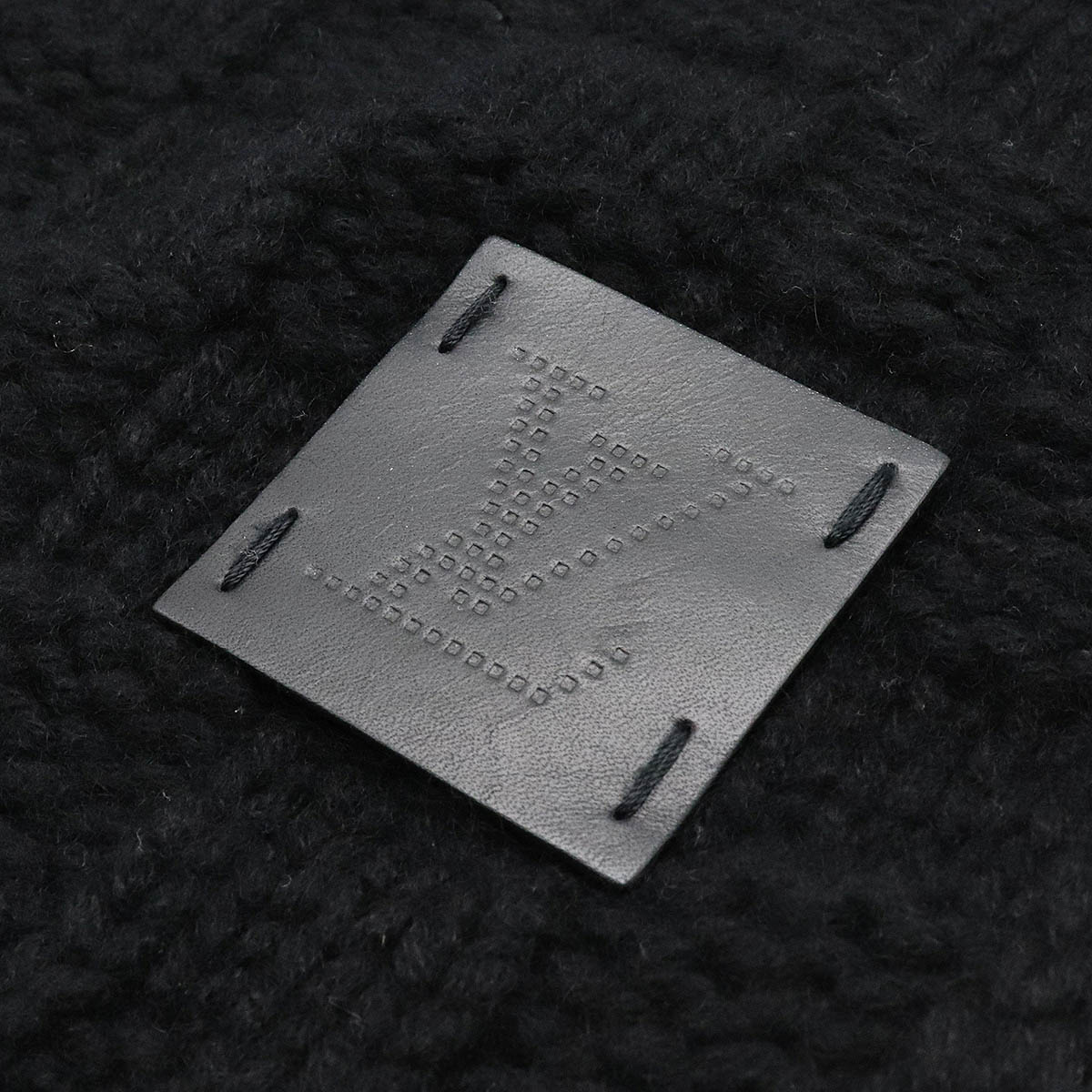
ルイ・ヴィトン LOUIS VUITTON エシャルプ ヘルシンキ マフラー M74421
(税込) 送料込み
商品の説明
商品説明
こちらの商品は質屋出品ですので、鑑定済みの本物の商品となります。詳しくは下記注意事項をご参照ください。詳細:ルイ・ヴィトンLOUISVUITTONエシャルプヘルシンキマフラーM74421
サイズ:約156cm×31cm
カラー:ノワール
素材:カシミヤ100%
付属品:箱(擦れ有、汚れ少)
備考:肌触りの良いカシミア100%を使用し、LVロゴが施されたレザーラベルがワンポイントとなったお洒落なマフラーです。2015年3週製造。若干の毛羽立ちが見られますが、目立つような大きな傷は無く、良い状態のお品となっております。
■状態:A+
商品番号:3.7.173028
22200円ルイ・ヴィトン LOUIS VUITTON エシャルプ ヘルシンキ マフラー M74421レディースファッション小物ルイヴィトンエシャルプ ヘルシンキ 黒 ユニセックス マフラー M74421◇ミウラ◇ ルイ・ヴィトン LOUIS VUITTON エシャルプ ヘルシンキ
ルイヴィトン LOUISVUITTON M74421 エシャルプヘルシンキ LVパッチ
楽天市場】◇ミウラ◇ルイ・ヴィトン LOUIS VUITTON エシャルプ
ルイヴィトンエシャルプ ヘルシンキ 黒 ユニセックス マフラー M74421
ルイヴィトンエシャルプ ヘルシンキ 黒 ユニセックス マフラー M74421
ルイヴィトンエシャルプ ヘルシンキ 黒 ユニセックス マフラー M74421
LOUIS VUITTON ルイヴィトン M74421 エシャルプ・ヘルシンキ カシミア
楽天市場】【アパレル】LOUIS VUITTON ルイ ヴィトン エシャルプ
◇ミウラ◇ ルイ・ヴィトン LOUIS VUITTON エシャルプ ヘルシンキ
中古・古着通販】LOUIS VUITTON (ルイ ヴィトン) エシャルプ
楽天市場】【アパレル】LOUIS VUITTON ルイ ヴィトン エシャルプ
ルイヴィトン LOUIS VUITTON エシャルプ ヘルシンキ M74421 ダミエ
ルイヴィトンエシャルプ ヘルシンキ 黒 ユニセックス マフラー M74421
LOUIS VUITTON - ルイヴィトン LOUIS VUITTON エシャルプ ヘルシンキ
ルイヴィトンエシャルプ ヘルシンキ 黒 ユニセックス マフラー M74421
中古・古着通販】LOUIS VUITTON (ルイ ヴィトン) エシャルプ
楽天市場】【フェブラリーバーゲン〇】【返品可】ルイヴィトン LOUIS
ルイ・ヴィトン LOUIS VUITTON エシャルプ ヘルシンキ マフラー M74421
楽天市場】【アパレル】LOUIS VUITTON ルイ ヴィトン エシャルプ
LOUIS VUITTON[ルイヴィトン] | エシャルプ ヘルシンキ カシミヤ
中古・古着通販】LOUIS VUITTON (ルイ ヴィトン) エシャルプ
LOUIS VUITTON - ルイヴィトン M74421 エシャルプヘルシンキ LVパッチ
ルイヴィトン LOUISVUITTON M74421 エシャルプヘルシンキ LVパッチ
ルイヴィトンエシャルプ ヘルシンキ 黒 ユニセックス マフラー M74421
開放倉庫 | 【中古】LOUIS VUITTON(ルイヴィトン)「エシャルプ
楽天市場】【フェブラリーバーゲン〇】【返品可】ルイヴィトン LOUIS
LOUISVUITTONスーパーコピー エシャルプヘルシンキ M74421 ノワール
楽天市場】【フェブラリーバーゲン〇】【返品可】ルイヴィトン LOUIS
LOUIS VUITTON(ルイヴィトン) / エシャルプ・ヘルシンキ/マフラー
ルイヴィトン エシャルプヘルシンキ カシミヤ LV ロゴ マフラー
LOUIS VUITTON(ルイヴィトン) / エシャルプ・ヘルシンキ/マフラー
LOUISVUITTONスーパーコピー エシャルプヘルシンキ M74421 ノワール
楽天市場】【アパレル】LOUIS VUITTON ルイ ヴィトン エシャルプ
LOUIS VUITTON(ルイヴィトン) / エシャルプ ヘルシンキ ノワール
Amazon | [ルイヴィトン] M74421 スカーフ エシャルプ ヘルシンキ
LOUIS VUITTON ルイヴィトン M74421 エシャルプ・ヘルシンキ カシミア
LOUISVUITTONスーパーコピー エシャルプヘルシンキ M74421 ノワール
楽天市場】【フェブラリーバーゲン〇】【返品可】ルイヴィトン LOUIS
LOUIS VUITTON(ルイヴィトン) / エシャルプ ヘルシンキ ノワール
楽天市場】【アパレル】LOUIS VUITTON ルイ ヴィトン エシャルプ
商品の情報
メルカリ安心への取り組み
お金は事務局に支払われ、評価後に振り込まれます
出品者
スピード発送
この出品者は平均24時間以内に発送しています










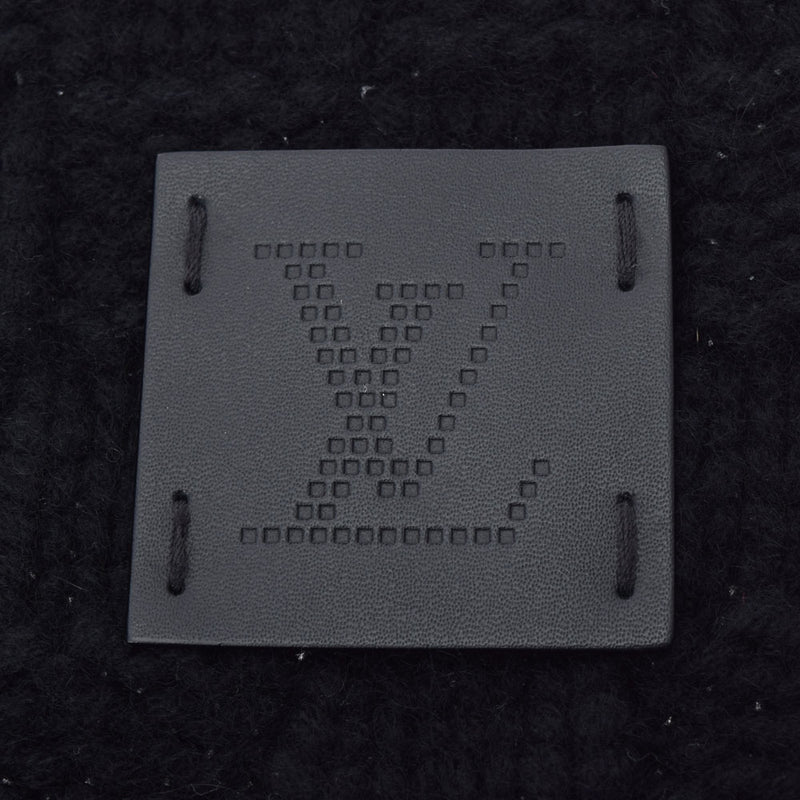






![LOUIS VUITTON[ルイヴィトン] | エシャルプ ヘルシンキ カシミヤ](https://boo-bee-2.s3-ap-northeast-1.amazonaws.com/upload/save_image/11181919_5a10093262cf3.jpg)













![Amazon | [ルイヴィトン] M74421 スカーフ エシャルプ ヘルシンキ](https://m.media-amazon.com/images/I/51ZDc6Ihn7L._AC_UY580_.jpg)