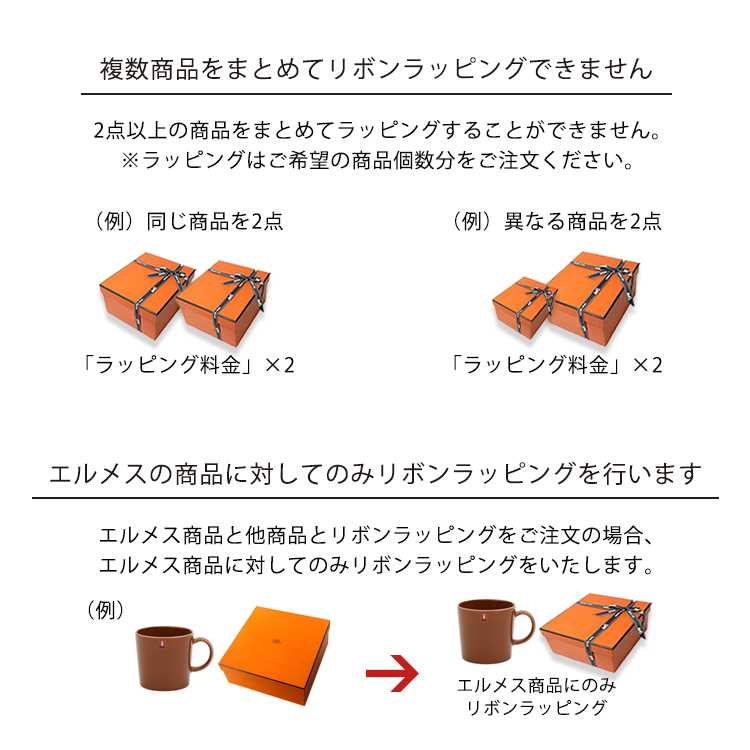
エルメス 値下げ!ラッピングリボン ロール 直径12.5cm
(税込) 送料込み
商品の説明
商品説明
エルメス【HERMES】ラッピングロールリボンですエルメスで商品を購入した時に付けて頂きました。
こちらは年号は入っておりません。
直径12.5cmです。
開封済みで何メートルか使用しました、使いさしです。
ネコポスにてお送りします。
返品、交換はできませんのでご了承下さい
24時間以内に受け取り通知をお願い致します。
6650円エルメス 値下げ!ラッピングリボン ロール 直径12.5cmインテリア/住まい/日用品オフィス用品エルメス 値下げ!ラッピングリボン ロール 直径14.5cm 購入格安 - wwwHermes - エルメス 値下げ!ラッピングリボン ロール 直径12.5cmの通販
Hermes - エルメス 値下げ!ラッピングリボン ロール 直径12.5cmの+
Hermes - エルメス 値下げ!ラッピングリボン ロール 直径12.5cmの+
エルメス 値下げ!ラッピングリボン ロール 直径14.5cm 購入格安 - www
Hermes - エルメス 値下げ!ラッピングリボン ロール 直径12.5cmの+
Hermes - エルメス 値下げ!ラッピングリボン ロール 直径12.5cmの+
エルメス 値下げ!ラッピングリボン ロール 2016年インテリア/住まい
エルメス 値下げ!ラッピングリボン ロール 直径14.5cmオフィス用品
その他エルメス リボンロール 新品セール - その他
エルメス 値下げ!ラッピングリボン ロール 直径14.5cm 購入格安 - www
エルメス 値下げ!ラッピングリボン 2017年 ロールラッピング/包装
エルメス 値下げ!ラッピングリボン ロール 直径14.5cm 購入格安 - www
【袋のみの購入不可】エルメス 純正紙袋 ショッパー ショッピングバッグ XLサイズ 有料 もれなくエルメスのリボンでラッピング ※必ずエルメス商品と一緒にご購入ください 袋のみの購入はキャンセルさせていただきます。 | Alevel(エイレベル)
お手軽価格で贈りやすい エルメス 値下げ!ラッピングリボン ロール
エルメス包装用リボン
Hermes - エルメス 値下げ!ラッピングリボン ロール 直径12.5cmの+
Hermes - エルメス 値下げ!ラッピングリボン ロール 直径12.5cmの通販
エルメス ラッピングリボンの+urbandrive.co.ke
その他エルメス リボンロール 新品セール - その他
Hermes - エルメス 値下げ!ラッピングリボン ロール 直径12.5cmの通販
Hermes - エルメス 値下げ!ラッピングリボン ロール 直径12.5cmの通販
Amazon.co.jp: 20色 300ヤード サテンリボン - シルクリボンロール18個
Hermes - エルメス 値下げ!ラッピングリボン ロール 直径12.5cmの通販
エルメス ラッピングリボンの+urbandrive.co.ke
エルメス包装用リボン
その他エルメス リボンロール 新品セール - その他
Hermes - エルメス 値下げ!ラッピングリボン ロール 直径12.5cmの通販
楽天市場】エルメス専用リボンラッピング : 輸入洋食器の専門店イデール
Hermes - エルメス 値下げ!ラッピングリボン ロール 直径12.5cmの通販
エルメス☆HERMES☆リボン☆巻き☆ロール☆年号なし - ショップ袋
Hermes - エルメス 値下げ!ラッピングリボン ロール 直径12.5cmの通販
Hermes - エルメス 値下げ!ラッピングリボン ロール 直径12.5cmの通販
エルメス(HERMES) エルメスロゴ入リボン掛け ※必ずエルメスの商品と一緒に御注文下さい
その他エルメス リボンロール 新品セール - その他
楽天市場】エルメス専用リボンラッピング : 輸入洋食器の専門店イデール
Hermes - エルメス 値下げ!ラッピングリボン ロール 直径12.5cmの通販
インテリア/住まい/日用品エルメス 値下げ!ラッピングリボン ロール
【楽天市場】エルメス専用リボンラッピング : 輸入洋食器の専門店
袋のみの購入不可】エルメス 純正紙袋 XLサイズ 有料 もれなくエルメス
Hermes - エルメス リボン セットの通販 by 🎀M's shop🎀|エルメス
商品の情報
メルカリ安心への取り組み
お金は事務局に支払われ、評価後に振り込まれます
出品者
スピード発送
この出品者は平均24時間以内に発送しています