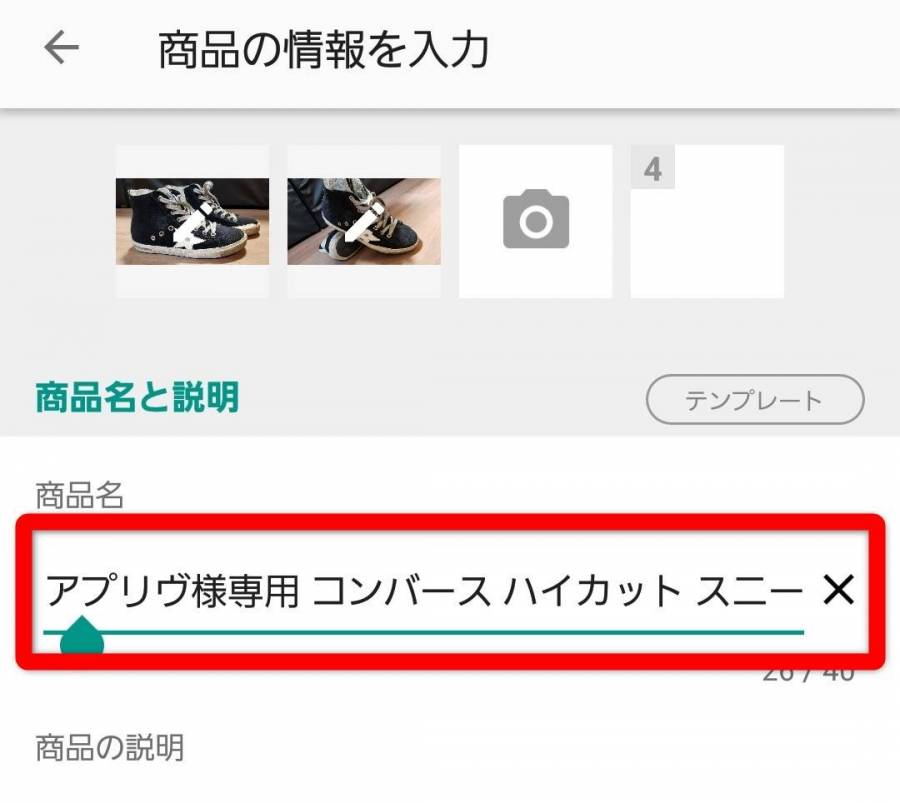
N♡♡様 専用ページ✩.*˚
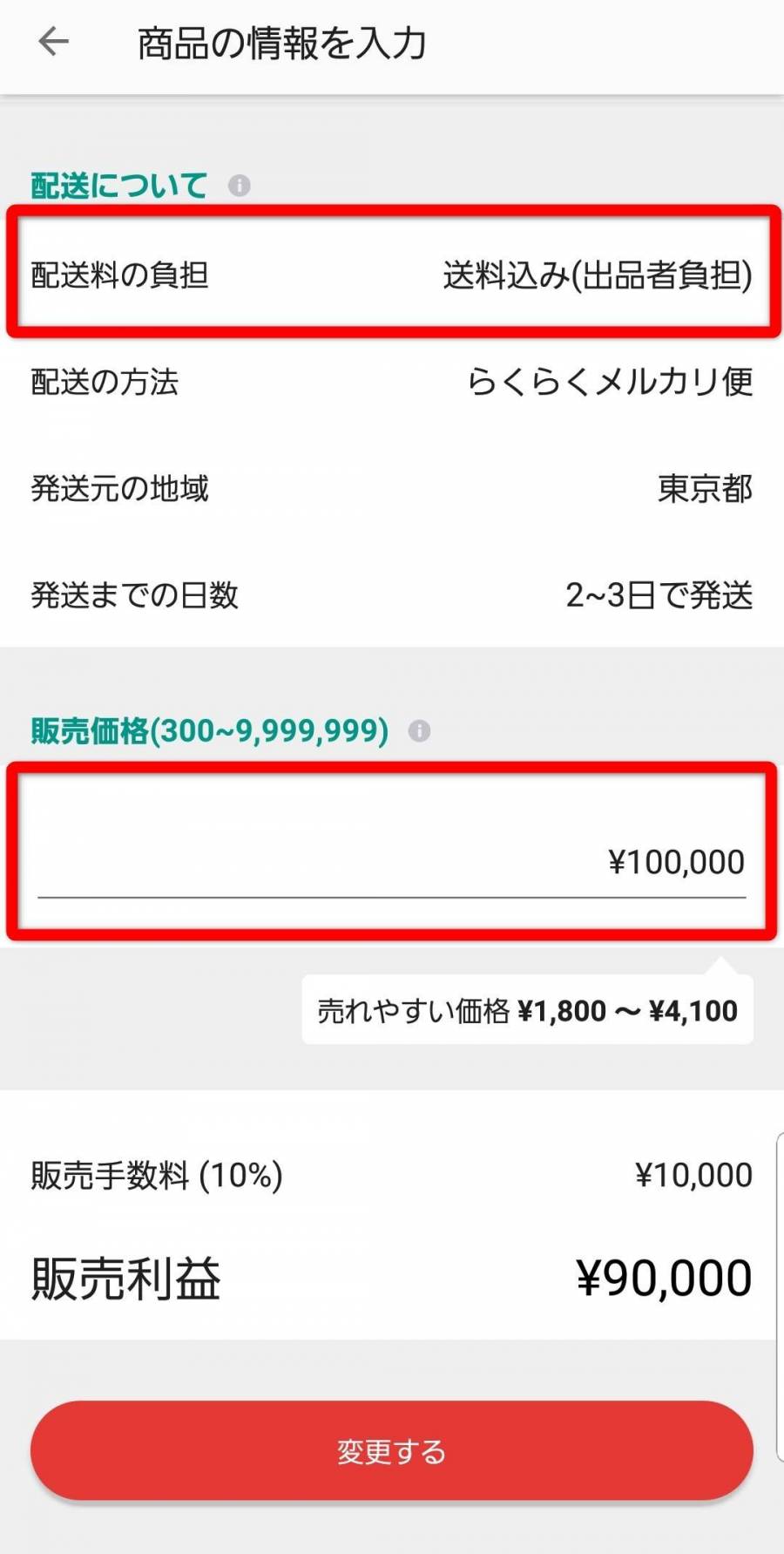
(税込) 送料込み
商品の説明
商品説明
♡フルオーダー5500円♡紛失分(700円)ミディアムオーバル6
4枚目画像参照
♡サイズ
↪︎3枚目画像参照
♡有料パーツ
↪︎ハートチャームオーロラ(600円)
イニシャルパーツH(500円)
クリア特殊ハートスワロ(450円)
ピンクハート指輪パーツ(700円)
♡キャラクター
↪︎ピアノちゃん
(※Hのイニシャルのピアノちゃんには王冠
Uのイニシャルのピアノちゃんには
ピンクのリボンを頭に乗せる)
♡発送
↪︎ご精算後、30日前後での発送
(※土日は作成不可のため日数に含まれません)
5915円N♡♡様 専用ページ✩.*˚コスメ/美容ネイルN様専用ページ - メルカリN.@様専用ページ - メルカリ
noko様専用
N.M様専用ページ】 | fée fée
N様専用ページ - メルカリ
N・A様 専用ページ 🎗の通販 by ange plaisir|ラクマ
N様専用ページ - メルカリ
N・A様 専用ページ 🎗の通販 by ange plaisir|ラクマ
N様専用ページ|Yahoo!フリマ(旧PayPayフリマ)
♡様専用ページ - メルカリ
N様専用ページ - メルカリ
N様専用ページ - メルカリ
S様 専用ページ - メルカリ
N・A様 専用ページ 🎗の通販 by ange plaisir|ラクマ
no様⦆専用ページの通販 by じぬ❤︎'s shop|ラクマ
Y 様 専用 ページ | Chigusa
N・A様 専用ページ 🎗の通販 by ange plaisir|ラクマ
N様専用ページ - メルカリ
E☆】様専用ページの通販 by nono's Shop|ラクマ
Hermes - エルメス 小物美品 フィルー ゴールドの+inforsante.fr
N・A様 専用ページ 🎗の通販 by ange plaisir|ラクマ
◼️ naaach☆様 ◼️専用ページ♡Sアート♡(メルカリ便) - メルカリ
みゃる様専用ページ♡-
jenny*様専用-
トラブルに注意】「メルカリ」専用ページの作り方と2つのデメリット
トラブルに注意】「メルカリ」専用ページの作り方と2つのデメリット
A様専用ページです😊
niconico☺︎様☆専用ページの通販 by p-p-p|ラクマ
トラブルに注意】「メルカリ」専用ページの作り方と2つのデメリット
Hermes - エルメス 小物美品 フィルー ゴールドの+inforsante.fr
ぽ様 専用ページ-
Hermes - エルメス 小物美品 フィルー ゴールドの+inforsante.fr
N.@様専用ページ - メルカリ
Hermes - エルメス 小物美品 フィルー ゴールドの+inforsante.fr
よーぐる様専用ページ うちわ文字 オーダー - メルカリ
トラブルに注意】「メルカリ」専用ページの作り方と2つのデメリット
Hermes - エルメス 小物美品 フィルー ゴールドの+inforsante.fr
E☆】様専用ページの通販 by nono's Shop|ラクマ
トラブルに注意】「メルカリ」専用ページの作り方と2つのデメリット
💗M∞R💗様専用ページの通販 by PIYO's shop|ラクマ
N様専用ページ - メルカリ
商品の情報
メルカリ安心への取り組み
お金は事務局に支払われ、評価後に振り込まれます
出品者
スピード発送
この出品者は平均24時間以内に発送しています