ビジョンピークス ワンポールテント TC
(税込) 送料込み
商品の説明
商品説明
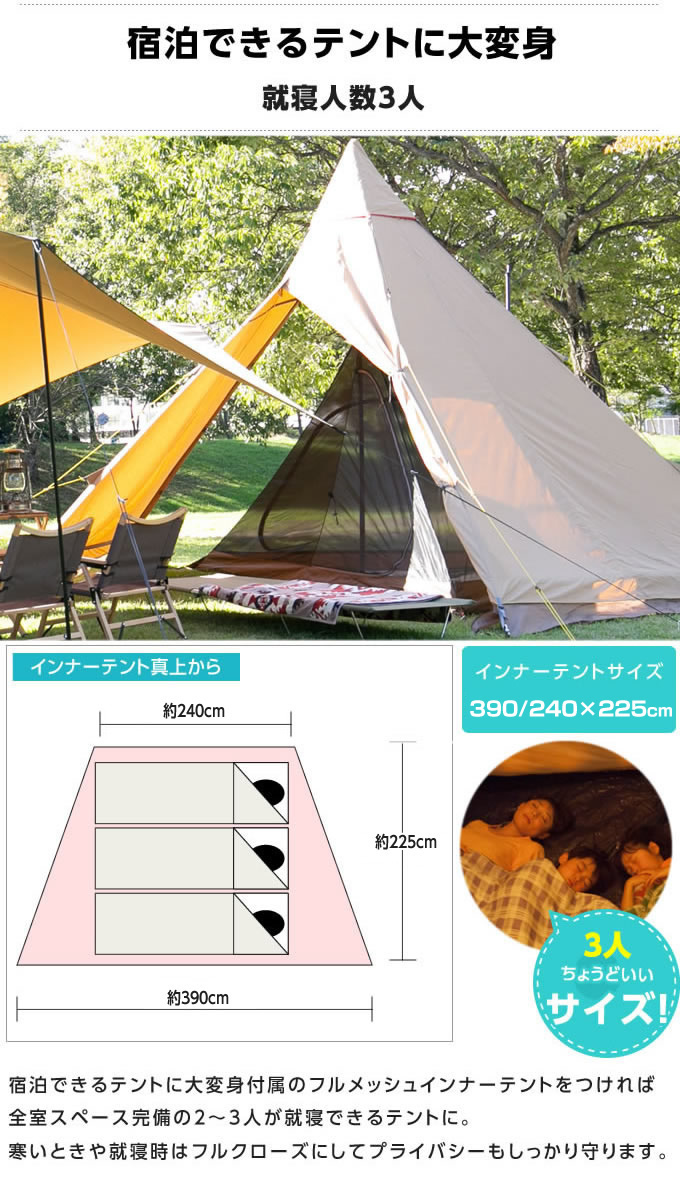
使用回数2回インナーテントは未使用遮光性と通気性を兼ね備えた綿混紡のシェルター
テントとしても使えるハーフインナーを標準装備。
■サイズ:約440×420×290(H)cm
■素材:
フライ/ポリエステル+綿
インナー/ポリエステルメッシュ
フロア/ポリエステルオックス
フレーム/スチール
■重量:約12.3kg
■収容人数:5人用
■就寝人数:3人用
■フロア耐水圧:2000mm
■原産国:ベトナム
■付属品:ペグ、ロープ、収納ケース
■特徴:ベンチレーション、設営時間15分
■原産国:中国、ベトナム
※原産国をお選びいただくことはできません
■使用上の注意:
通常のテント素材に比べ、綿混紡は火の粉による穴があきにくい素材ですが、防炎加工ではございませんので、シェルター内あるいはシェルター近くでの焚き火はご遠慮ください。
雨水等が集中すると雨漏り等の可能性がありますので、しっかり水が流れるようにロープを張ってご使用ください。
また荒天の際は強い雨に耐えきれず浸水する事がありますので使用を中止してください。
使用後は十分に乾燥させてから高温多湿の場所を避けて保管してください。
乾燥が不十分な状態で保管されますとカビや悪臭の原因となりますのでご注意ください。
#ワンポールテント
#ポリコットン
10920円ビジョンピークス ワンポールテント TCスポーツ/アウトドアアウトドアビジョンピークス(VISIONPEAKS) テント ワンポールテント TCティピビジョンピークス(VISIONPEAKS) テント ワンポールテント TCティピ
ビジョンピークス(VISIONPEAKS) テント ワンポールテント TCティピ
最善 ビジョンピークス TCティピーシェルター ワンポールテント | www
ビジョンピークス(VISIONPEAKS) テント ワンポールテント TCティピ
新色追加! 新品 ビジョンピークス ワンポールテント TCティピ
ワンポールテント TCティピシェルター VP160101I01 ビジョンピークス
ビジョンピークス(VISIONPEAKS) テント ワンポールテント TCティピ
ビジョンピークスTCティピ ワンポールテント&フタマタノサソイ
ビジョンピークス ワンポールテント サーカスTCと同サイズキャンプ
あなたにおすすめの商品 VISIONPEAKS◇TC TIPI SHELTERティピ
ビジョンピークス] ワンポールテント 1~3人用 TCティピシェルター
ビジョンピークス ワンポールテント サーカスTCと同サイズキャンプ
ショッピング最安 ビジョンピークス ワンポールテント TC - アウトドア
楽天市場】テント ワンポールテント TCティピシェルター インナー付
ビジョンピークス ワンポールテント - テント
ビジョンピークス(VISIONPEAKS) テント ワンポールテント TCティピ
ビジョンピークス ワンポールテント - テント
でおすすめアイテム。 ワンポールテント TCティピ テント 【おまけ付き
ビジョンピークス] ワンポールテント 1~3人用 TCティピシェルター
アウトドアビジョンピークス TCティピシェルター - テント/タープ
【HOW TO アウトドア】TCティピーシェルター設営動画 (道具紹介)
Amazon | [ビジョンピークス] ワンポールテント 1~3人用 TCティピ
最大級の通販サイト VISIONPEAKS◇テント/ワンポール/2~3人用/CML/TC
ビジョンピークス ティピ シェルター ワンポールテント tc-
ビジョンピークス ワンポールテント tc - テント/タープ
ビジョンピークス ワンポールテント TCティピシェルター インナー付
テント ワンポールテント TCティピシェルター インナー付セット 2~3人用 VP160101I01 ティピテント ビジョンピークス VISIONPEAKS
新品・未開封 ビジョンピークス ワンポールテント TCティピシェルター
ビジョンピークス ワンポールテント TCティピシェルター インナー付セット-
ホット ビジョンピークステント ワンポールテントTCティピシェルター
ビジョンピークス ワンポールテント - テント
新色追加! 新品 ビジョンピークス ワンポールテント TCティピ
売り取扱店 ビジョンピークス ワンポールテント サーカスTCと同
最新発見 新品!ビジョンピークス TCティピシェルター サーカスTCワン
楽天市場】テント ワンポールテント TCティピシェルター インナー付
アウトドアビジョンピークス TCティピシェルター - テント/タープ
ビジョンピークスのワンポールテントレビュー、サーカスTCが買えない
VISIONPEAKS(ビジョンピークス) / TCティピシェルター テント/ワン
HOW TO アウトドア】新TCティピーシェルター設営動画 (道具紹介
ビジョンピークスのTCティピシェルター | 好きなキャンプギアに囲まれたい
商品の情報
メルカリ安心への取り組み
お金は事務局に支払われ、評価後に振り込まれます
出品者
スピード発送
この出品者は平均24時間以内に発送しています











![ビジョンピークス] ワンポールテント 1~3人用 TCティピシェルター](https://auctions.c.yimg.jp/images.auctions.yahoo.co.jp/image/dr000/auc0409/users/3428ff355104811d383903e9a47ab1f62e57b534/i-img1000x1200-1695641390814rwffbf.jpg)







![ビジョンピークス] ワンポールテント 1~3人用 TCティピシェルター](https://auctions.c.yimg.jp/images.auctions.yahoo.co.jp/image/dr000/auc0411/users/10d6b59c0a9abf37435d95d40ae6226e69a377e1/i-img1007x1200-1700718013439myi2s5.jpg)


![Amazon | [ビジョンピークス] ワンポールテント 1~3人用 TCティピ](https://m.media-amazon.com/images/I/710aq04BhKL._AC_UF350,350_QL80_.jpg)