新品未使用!エルメス ツイリー hermes twilly wow
(税込) 送料込み
商品の説明
商品説明
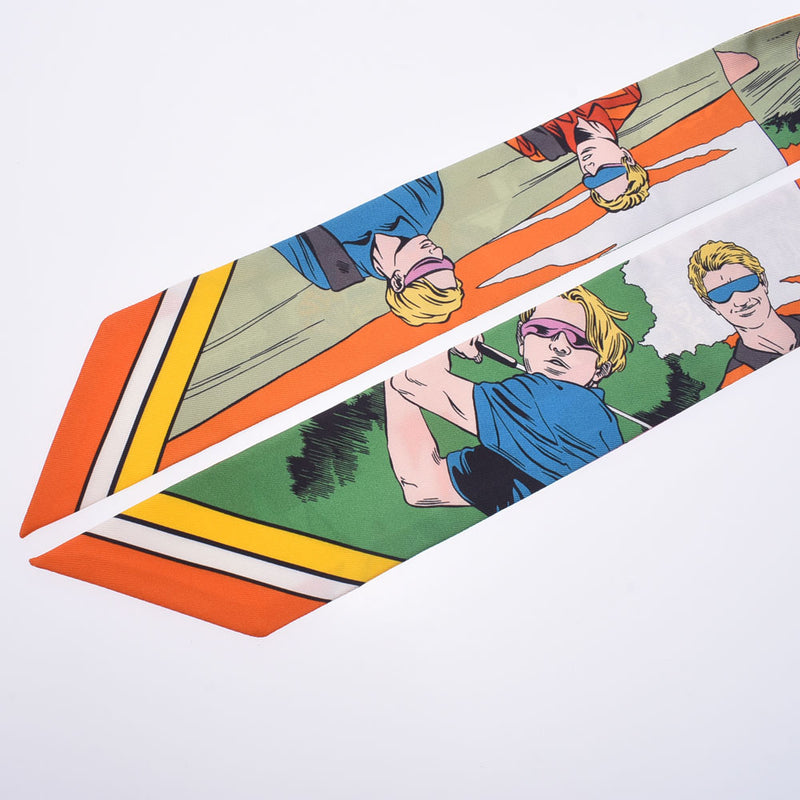
エルメスツイリーワオhermestwillydresswow
アメコミ風の柄がエルメスでは珍しく、気に入って購入しましたが使用しないまま
でしたので、未使用のうちに出品します。
ジャポンタグ付きです。お箱はついておりません。
ご希望の場合は+500円でお付けすることが可能です。
購入店舗カードは付き合いがございますのでお付けできません。
【付属品】
ジャポンタグ
すり替え防止のため返品返金はできません。
必ずご不明点はご購入前にご質問ください。
#hermes
#dior
#yori
#twilly
#エルメス
#エルメスツイリー
14700円新品未使用!エルメス ツイリー hermes twilly wowレディースファッション小物ファッション小物HERMES エルメス ツイリー WOW - バンダナ/スカーフファッション小物HERMES エルメス ツイリー WOW - バンダナ/スカーフ
ファッション小物HERMES エルメス ツイリー WOW - バンダナ/スカーフ
エルメス新品未使用!エルメス ツイリー hermes twilly wow - www
ファッション小物HERMES エルメス ツイリー WOW - バンダナ/スカーフ
ファッション小物HERMES エルメス ツイリー WOW - バンダナ/スカーフ
新品・未使用品】エルメス HERMES ツイリー WOW アメコミ柄 リボン
ファッション小物HERMES エルメス ツイリー WOW - バンダナ/スカーフ
逆輸入 HERMES エルメス ツイリー WOW バンダナ/スカーフ - kotor.travel
ファッション小物HERMES エルメス ツイリー WOW - バンダナ/スカーフ
国内最安値! 【KOMEHYO】【未使用品】エルメス エルメス WOW 063463S
Hermes - エルメス ツイリー WOW アメコミ柄 リボン スカーフ シルク
Hermes - □新品□未使用□ HERMES エルメス WOW シルク100% ツイリー
エルメス ツイリー WOW ワォ ピンク マルチカラー ブルー レッド
新品・未使用品】エルメス HERMES ツイリー WOW アメコミ柄 リボン
Hermes - エルメス ツイリー WOW アメコミ柄 リボン スカーフ シルク
エルメス 未使用品 ツイリー WOW 063463S 16 アメコミ系 スカーフ
WEB正規販売店 HERMES エルメス スカーフ ツイリー アメコミ風 - 小物
2021年 春夏 新作 HERMES エルメス ツイリー スカーフ
ファッション小物HERMES エルメス ツイリー WOW - バンダナ/スカーフ
エルメスツイリー WOW アメコミ風イラスト エクスリブリス/Ex-libris
エルメス 未使用品 ツイリー WOW 063463S 16 アメコミ系 スカーフ
エルメス ドレス バンダナ/スカーフ(レディース)の通販 68点 | Hermes
お得なクーポン配布中 エルメス ツイリー2本新品未使用品 - 小物
Hermes - 【新品未使用】エルメスツイリーWOW 2021SSの通販 by pupu's
新品 エルメス ツイリー スペースダービー-
新品未使用】HERMES ツイリー ピンク系-
エルメス 未使用品 ツイリー WOW 063463S 16 アメコミ系 スカーフ
エルメス新品未使用!エルメス ツイリー hermes twilly wow - www
新品未使用 エルメス ツイリー 2本セット wow 2021年 美品-
未使用 HERMES エルメス ツイリー 2本セット-
ファッション小物HERMES エルメス ツイリー WOW - バンダナ/スカーフ
在庫あり】 エルメス ツイリー スカーフ 新品未使用 バンダナ/スカーフ
エルメス ツイリー 2023SS 自由に アンリベルテ シルク100% 箱付き 未
Hermes - 未使用品 エルメス ツイリー リボンスカーフ WOW 063463S
HERMES Twilly エルメス ツイリー-
Sale】エルメス ツイリー 2022春夏 GRAND THEATRE NOUVEAU グラン
新品・未使用品)エルメス HERMES ツイリー WOW アメコミ柄 リボン
エルメスツイリー WOW マルチカラー レディース スカーフ HERMES
未使用極美品☆ エルメス HERMES ツイリー TWILLY ワオ WOW スカーフ
Hermes - HERMES エルメス ツイリー 空飛ぶカレ 黒の+inforsante.fr
商品の情報
メルカリ安心への取り組み
お金は事務局に支払われ、評価後に振り込まれます
出品者
スピード発送
この出品者は平均24時間以内に発送しています