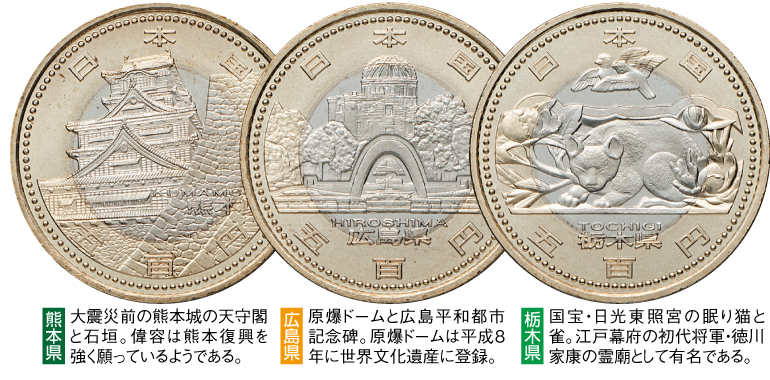
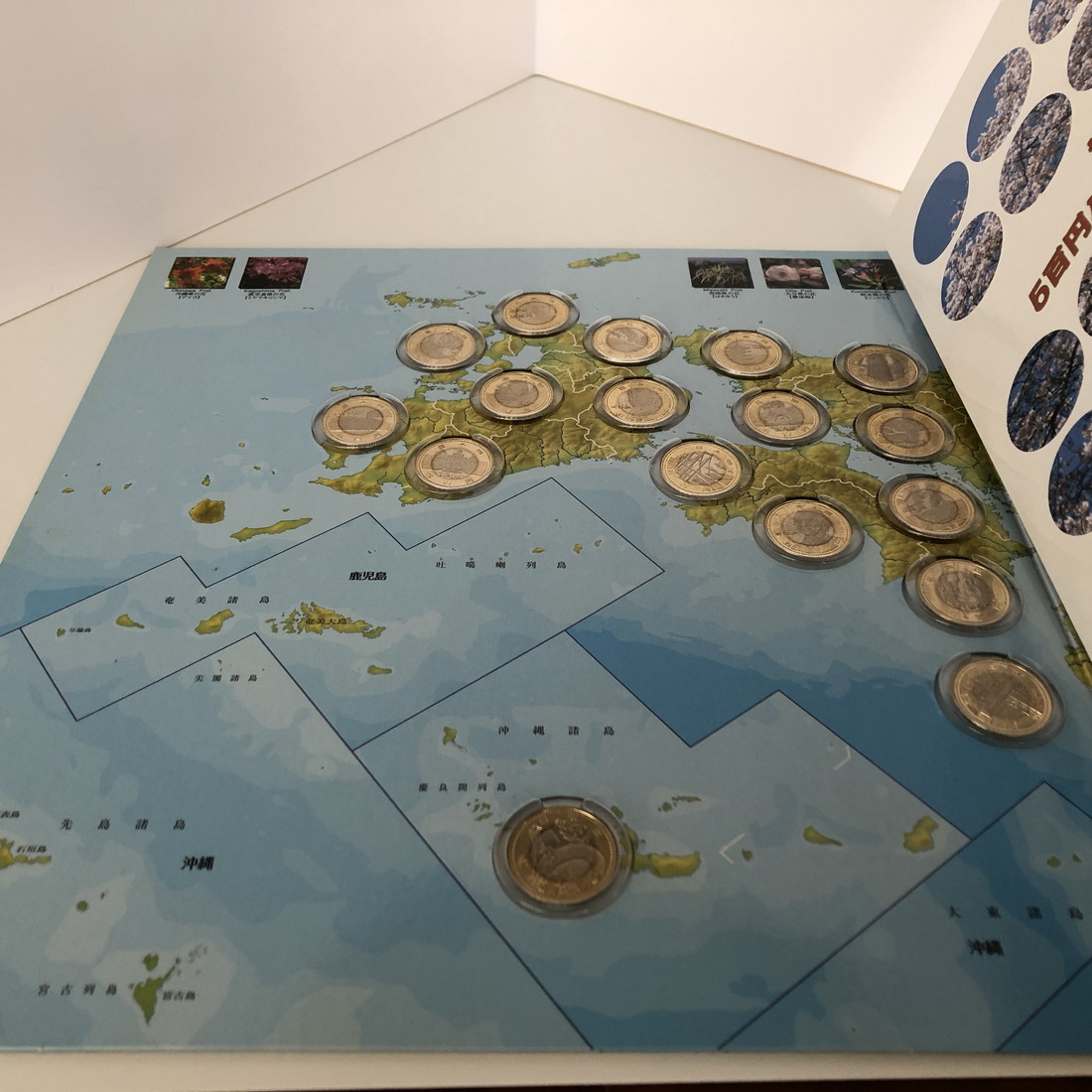
虎次郎様・専用地方自治500円・47都道府県セットカード式 バインダー付き
(税込) 送料込み
商品の説明
商品説明
虎次郎様専用です。他の方のご購入はお控えいただきますようお願いいたします。
地方自治施行60周年500円バイカラークラッド・カード式専用バインダー付きの
47都道府県コンプリートセットです。
22200円虎次郎様・専用地方自治500円・47都道府県セットカード式 バインダー付きエンタメ/ホビー美術品/アンティーク地方自治法施行60周年記念貨幣『五百円記念貨47都道府県セット地方自治・500円 47都道府県フルセット!!(カード式タイプ)-
地方自治 60周年記念500円バイカラー・クラッド貨幣カード 47都道府県
地方自治施行60 47都道府県バインダー付セット-
地方自治法施行60周年記念貨幣『五百円記念貨47都道府県セット
地方自治施行60 47都道府県バインダー付セット-
地方自治施行60 47都道府県バインダー付セット-
虎次郎様専用 - リール
地方自治・500円 47都道府県フルセット!!(カード式タイプ)-
地方自治法施行60周年記念貨幣『五百円記念貨47都道府県セット
地方自治・500円 47都道府県フルセット!!(カード式タイプ)-
500円 地方自治 47都道府県 バインダーの値段と価格推移は?|7件の
虎次郎様専用 - リール
地方自治法施行60周年記念 500円 47都道府県 バイカラークラッド貨幣
地方自治法施行60周年記念貨幣『五百円記念貨47都道府県セット
販売 ☆明治6年(大特年)☆美品 medpraktik.com
Yahoo!オークション -「地方自治 500円 バインダー」(記念硬貨) (日本
地方自治法施行60周年記念 500円 47都道府県 バイカラークラッド貨幣
スーパーセール 中国 古錢 銅幣 壹圓 宣統三年 大清銀幣 - 美術品
スーパーセール 中国 古錢 銅幣 壹圓 宣統三年 大清銀幣 - 美術品
楽天市場】地方自治法施行60周年記念 500円バイカラー・クラッド貨幣
Yahoo!オークション -「47都道府県 500円」(記念硬貨) (日本)の落札
スーパーセール 中国 古錢 銅幣 壹圓 宣統三年 大清銀幣 - 美術品
地方自治法施行60周年記念貨幣『五百円記念貨47都道府県セット
Yahoo!オークション -「地方自治 500円 バインダー」の落札相場・落札価格
楽天市場】地方自治法施行60周年記念 500円バイカラー・クラッド貨幣
相場 PCー8801mk2グラフィック・ワークブック ビジュアル体験
2024年最新】Yahoo!オークション -地方自治 500円 バインダーの中古品
地方自治法施行60周年記念 五百円バイカラー・クラッド貨幣収納
2024年最新】地方自治 バインダーの人気アイテム - メルカリ
500円 地方自治 47都道府県 バインダーの値段と価格推移は?|7件の
地方自治法施行60周年記念 500円 47都道府県 バイカラークラッド貨幣
楽天市場】地方自治法施行60周年記念 500円バイカラー・クラッド貨幣
Yahoo!オークション -「47都道府県 500円」(貨幣) の落札相場・落札価格
地方自治法施行60周年記念 500円 47都道府県 バイカラークラッド貨幣
スーパーセール 中国 古錢 銅幣 壹圓 宣統三年 大清銀幣 - 美術品
2024年最新】500円 都道府県 記念硬貨の人気アイテム - メルカリ
楽天市場】地方自治法施行60周年記念 500円バイカラー・クラッド貨幣
Yahoo!オークション -「地方自治 500円 バインダー」の落札相場・落札価格
地方自治法施行60周年記念貨幣『五百円記念貨47都道府県セット
2024年最新】Yahoo!オークション -地方自治 500円 バインダーの中古品
商品の情報
メルカリ安心への取り組み
お金は事務局に支払われ、評価後に振り込まれます
出品者
スピード発送
この出品者は平均24時間以内に発送しています